Isotonic Solution: Imagine you’re in the hospital and the nurse hooks you up to an IV. What is in that IV? Is it just water? No way. If you were pumped full of pure water, your blood cells would burst. How horrible would that be? That IV is full of saline, a liquid with the same concentration of solutes as your blood cells. Why is this important? Because you want your blood cells to sit in an isotonic solution.
An isotonic solution is when two solutions, separated by a semipermeable membrane, have equal concentrations of solutes and water. Imagine you’re at a party and there is an equal number of guests in the living room and in the kitchen. It doesn’t make much of a difference where you stand because you are equally as comfortable in either room. You have just as much space, you can move just as easily, and you have equal access to food. You don’t spend a lot of energy trying to get out of one room or into another. This party is like an isotonic solution; everything is equal from room to room.
Now, compare this with a party where the living room is packed full of guests, while there are only a few in the kitchen. I don’t know about you, but I would be trying to get to the kitchen as fast as possible. The concentration in each room is different, so people are moving around trying to equal things out. Nature likes equality and that is apparent when it comes to solutions.
Isotonic Solution Definition
An isotonic solution is one that has the same osmolarity, or solute concentration, as another solution. If these two solutions are separated by a semipermeable membrane, water will flow in equal parts out of each solution and into the other. The effect is zero water flow between the two solutions, although water is moving both ways.
In biology, some cells must be maintained in an isotonic solution to support cellular functions. Many animal cells, which lack a cell wall to provide support against the effects of water pressure, rely on the stability of the external environment to maintain their shape. Most animals maintain the pH and osmolarity of the fluids inside of their bodies to create isotonic solutions to bathe their cells. This solution can carry nutrients and water, but only in proportions equal to that inside the cell.
A depiction of a cell in an isotonic solution can be seen above. Note that because there is the same concentration of solute molecules inside and outside of the cell water molecules are simply exchanged through the cell membrane. This can be contrasted to the effects of a hypertonic solution, in which water molecules leave the cell, or a hypotonic solution in which water enters the cell.
What is an example of an isotonic solution?
Sometimes, potassium chloride can be included in the solution. An isotonic solution is a ‘solution that has the same salt concentration as cells and blood.’ … One example of an isotonic solution is the intravenously infused fluids in hospitalized patients. Some of these solutions are 0.9% NaCI or 5 percent dextrose.
What are the isotonic fluids?
Isotonic sodium chloride solution (normal saline [NS]) and lactated Ringer (LR) solution are isotonic crystalloid fluids, the standard intravenous (IV) fluids used for initial volume resuscitation. Another crystalloid solution used is Plasmalyte. These solutions expand the intravascular and interstitial fluid spaces.
What are isotonic hypertonic and hypotonic solutions?
What Is An Isotonic Solution
Tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a semipermeable membrane cell membrane. In other words, tonicity is the relative concentration of solutes dissolved in solution which determines the direction and extent of diffusion. It is commonly used when describing the response of cells immersed in an external solution.
Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without the net solvent movement. It is also a factor affecting imbibition.
A hypertonic solution has a greater concentration of solutes than another solution. In biology, the tonicity of a solution usually refers to its solute concentration relative to that of another solution on the opposite side of a cell membrane; a solution outside of a cell is called hypertonic if it has a greater concentration of solutes than the cytosol inside the cell. When a cell is immersed in a hypertonic solution, osmotic pressure tends to force water to flow out of the cell in order to balance the concentrations of the solutes on either side of the cell membrane.
The cytosol is conversely categorized as hypotonic, the opposite of the outer solution.
When plant cells are in a hypertonic solution, the flexible cell membrane pulls away from the rigid cell wall but remains joined to the cell wall at points called plasmodesmata. The cells often take on the appearance of a pincushion, and the plasmodesmata almost cease to function because they become constricted, a condition known as plasmolysis. In-plant cells the terms isotonic, hypotonic, and hypertonic cannot strictly be used accurately because the pressure exerted by the cell wall significantly affects the osmotic equilibrium point.
Read Also: What Is Specific Volume?
Some organisms have evolved intricate methods of circumventing hypertonicity. For example, saltwater is hypertonic to the fish that live in it. Because the fish need a large surface area in their gills in contact with seawater for gas exchange, they lose water osmotically to the sea from gill cells. They respond to the loss by drinking large amounts of salt water and actively excreting the excess salt. This process is called osmoregulation.
Define Isotonic Solution
Isotonic Solution Examples
Blood Cells
When the plasma surrounding blood cells is an isotonic solution, compared to the solution inside the blood cells, the cells function normally. The isotonic solution allows the cells to move water and nutrients in and out of the cells. This is necessary for blood cells to perform their function of delivering oxygen and other nutrients to other parts of the body. If the cells are in a hypertonic environment, they will become plasmolyzed and will not contain enough water to perform cellular functions. If the cells exist in a hypotonic environment, they will lyse, spilling their contents into the bloodstream. This can cause dangerous side effects, as well as the loss of many blood cells. These events can be seen in the graphic below.
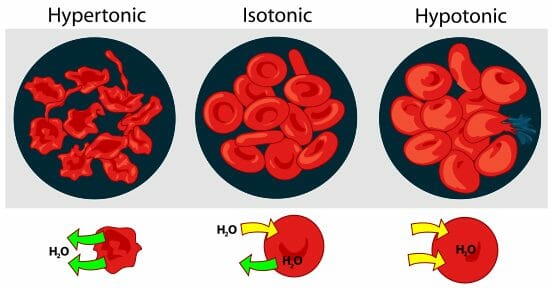
To avoid either of the negative situations from happening during the transfusion of nutrients and medicine, the solution that carries the medicine must be an isotonic solution, compared to the patient’s blood. The osmolarity of the IV fluid can be adjusted using special salts and sugars that act simply as solutes to dilute or strengthen a substance. Once a medicine is an isotonic solution compared to the blood, it can be added through an IV and no damage will occur to blood cells.
Osmoconformers and Osmoregulators
In nature, there are two types of organisms: those that conform to the osmolarity of the environment, and those that regulate the osmolarity of their body to be different from the environment. The first is known as osmoconformers and have evolved to have cells that match the osmolarity of the environment. These animals always exist in an isotonic solution, because they have evolved to be the same concentration as the environment. This condition can be seen in many of the “lower” forms of life such as the sea slugs, coral, and jellyfish. The other group, the osmoregulatory, does not exist in an isotonic environment.
This means that water tends to want to enter or leave their bodies, and they have various methods for dealing with this. However, inside of an osmoregulatory, the cells will still exist in an isotonic solution, as the organism needs its cells to remain functional. Both osmoregulatory and osmoconformers have different benefits for conducting life the way they do, but an isotonic solution is usually created around cells.